Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 /GaN interfaces with suppressed Ga-oxide interlayer via sputter deposition of SiO
/GaN interfaces with suppressed Ga-oxide interlayer via sputter deposition of SiO
Onishi, Kentaro*; Kobayashi, Takuma*; Mizobata, Hidetoshi*; Nozaki, Mikito*; Yoshigoe, Akitaka; Shimura, Takayoshi*; Watanabe, Heiji*
Japanese Journal of Applied Physics, 62(5), p.050903_1 - 050903_4, 2023/05
While the formation of an GaO interlayer is key to achieving SiO
interlayer is key to achieving SiO /GaN interfaces with low defect density, it can affect the reliability and stability of metal-oxide-semiconductor (MOS) devices if the annealing conditions are not properly designed. In the present study, we aimed to minimize the growth of the GaO
/GaN interfaces with low defect density, it can affect the reliability and stability of metal-oxide-semiconductor (MOS) devices if the annealing conditions are not properly designed. In the present study, we aimed to minimize the growth of the GaO layer on the basis of the sputter deposition of SiO
layer on the basis of the sputter deposition of SiO on GaN. Synchrotron radiation X-ray photoelectron spectrometry measurements confirmed the suppressed growth of the GaO
on GaN. Synchrotron radiation X-ray photoelectron spectrometry measurements confirmed the suppressed growth of the GaO layer compared with a SiO
layer compared with a SiO /GaN structure formed by plasma-enhanced chemical vapor deposition. Negligible GaO
/GaN structure formed by plasma-enhanced chemical vapor deposition. Negligible GaO growth was also observed when subsequent oxygen annealing up to 600
growth was also observed when subsequent oxygen annealing up to 600 C was performed. A MOS device with negligible capacitance-voltage hysteresis, nearly ideal flat-band voltage, and low leakage current was demonstrated by performing oxygen and forming gas annealing at temperatures of 600
C was performed. A MOS device with negligible capacitance-voltage hysteresis, nearly ideal flat-band voltage, and low leakage current was demonstrated by performing oxygen and forming gas annealing at temperatures of 600 C and 400
C and 400 C, respectively.
C, respectively.
Miyakawa, Kazuya
JAEA-Data/Code 2021-021, 23 Pages, 2022/03
In the Horonobe underground research laboratory (HURL) project, groundwater chemistry was analyzed to investigate changes due to the excavation of the underground facility and to review geochemical models until the fiscal year 2019. From the fiscal year 2020, to proceed remaining important issues deduced from the conclusion of the investigations during the fiscal year 2015-2019, primary data such as groundwater chemistry need to be successively acquired. Here, the chemical analysis of 54 groundwater samples in 2021 from boreholes drilled in the 140 m, 250 m, 350 m gallery in the HURL, and water rings settled in three vertical shafts is presented. Analytical results include groundwater chemistry such as physicochemical parameters (pH, electrical conductivity), dissolved ions (Na , K
, K , Li
, Li , NH
, NH
 , Cl
, Cl , Br
, Br , NO
, NO
 , SO
, SO
 , PO
, PO
 , Ca
, Ca , Mg
, Mg , Sr
, Sr , P, Total-Mn, Si, Total-Fe, Al, B, F
, P, Total-Mn, Si, Total-Fe, Al, B, F , I
, I , alkalinity, total organic carbon, total inorganic carbon, CO
, alkalinity, total organic carbon, total inorganic carbon, CO
 , HCO
, HCO
 , Ba, Fe
, Ba, Fe , sulfide),
, sulfide), 
 O,
O,  D, and tritium content along with a detailed description of analytical methods.
D, and tritium content along with a detailed description of analytical methods.
Tobita, Minoru*; Haraga, Tomoko; Sasaki, Takayuki*; Seki, Kotaro*; Omori, Hiroyuki*; Kochiyama, Mami; Shimomura, Yusuke; Ishimori, Kenichiro; Kameo, Yutaka
JAEA-Data/Code 2019-016, 72 Pages, 2020/02
In the future, radioactive wastes which generated from research and testing reactors in Japan Atomic Energy Agency are planning to be buried for the near surface disposal. Therefore, it is required to establish the method to evaluate the radioactivity concentrations of radioactive wastes by the time it starts disposal. In order to contribute to this work, we collected and analyzed the samples generated from JRR-2, JRR-3 and Hot laboratory facilities. In this report, we summarized the radioactivity concentrations of 25 radionuclides ( H,
H,  C,
C, 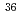 Cl,
Cl,  Co,
Co, 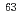 Ni,
Ni,  Sr,
Sr, 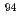 Nb,
Nb, 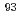 Mo,
Mo,  Tc,
Tc, 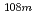 Ag,
Ag,  Sn,
Sn,  I,
I,  Cs,
Cs, 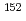 Eu,
Eu, 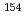 Eu,
Eu,  U,
U,  U,
U,  U,
U,  Pu,
Pu,  Pu,
Pu,  Pu,
Pu,  Pu,
Pu,  Am,
Am,  Am,
Am,  Cm) which were obtained from radiochemical analysis of those samples.
Cm) which were obtained from radiochemical analysis of those samples.
Sato, Yoshiyuki; Tanaka, Kiwamu; Ueno, Takashi; Ishimori, Kenichiro; Kameo, Yutaka
Hoken Butsuri, 51(4), p.209 - 217, 2016/12
A large amount of contaminated rubbles were generated by the accident at the Fukushima Daiichi Nuclear Power Station (F1NPS). For safe decommissioning of F1NPS, it is important to evaluate the composition and concentration of radionuclides in the rubbles. In this paper, to characterize the rubbles collected at F1NPS in Unit-1, Unit-2 and Unit-3, radiochemical analysis was operated. As a result of radiochemical analysis,  -ray-emitting nuclides
-ray-emitting nuclides  Co,
Co,  Cs and
Cs and 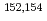 Eu,
Eu,  -ray-emitting nuclides
-ray-emitting nuclides  H,
H,  C,
C,  Sr and
Sr and  Tc, and
Tc, and  -particle-emitting nuclides
-particle-emitting nuclides 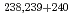 Pu,
Pu,  Am and
Am and  Cm were detected. In contrast,
Cm were detected. In contrast, 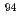 Nb and
Nb and 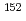 Eu concentrations were below the detection limit. Measured radioactive concentrations implied that
Eu concentrations were below the detection limit. Measured radioactive concentrations implied that  H,
H,  C,
C,  Co and
Co and  Sr concentrations depended on
Sr concentrations depended on  Cs concentration respectively. This analysis was characterized the radioactivity concentrations of the rubbles.
Cs concentration respectively. This analysis was characterized the radioactivity concentrations of the rubbles.
Shinohara, Nobuo; Kono, Nobuaki; Suyama, Kenya; Inagawa, Jun; Nakahara, Yoshinori; Kurosawa, Setsumi; Watanabe, Kazuo; Usuda, Shigekazu; Oshima, Masumi; Katsuta, Hiroji; et al.
Radiochimica Acta, 89(3), p.135 - 138, 2001/05
Times Cited Count:2 Percentile:19.66(Chemistry, Inorganic & Nuclear)no abstracts in English
Adachi, Takeo
EUR-19943-EN, p.211 - 215, 2001/00
no abstracts in English
JNC TN8400 2000-030, 17 Pages, 2000/12
As a representative of natural marine groundwater, the author selected pumped water from a Quaternary sedimentary aquifer of the Mobara gas-field in Japan and measured the concentration of total organic carbon (TOC) and of organic acid anions (formic, acetic, lactic, succinic, humic, fulvic, propionic, valeric and butyric acids). The concentration of TOC ranged from 22 1 to 24
1 to 24 0mg/L. As organic acid anions, only succinic and fulvic acids were detected and each concentration was given to be from 5.8
0mg/L. As organic acid anions, only succinic and fulvic acids were detected and each concentration was given to be from 5.8 0.5 to 8.3
0.5 to 8.3 0.3 and from 3.3
0.3 and from 3.3 0.2 to 3.5
0.2 to 3.5 0.2mg/L, respectively. By consideration of the temperature and the [SO
0.2mg/L, respectively. By consideration of the temperature and the [SO
 ] of the groundwater, it is inferred that the organic acid has been significantly decomposed by activities of microbes, such as the fermentation process, CH
] of the groundwater, it is inferred that the organic acid has been significantly decomposed by activities of microbes, such as the fermentation process, CH COO
COO + H
+ H O = HCO
O = HCO
 + CH
+ CH .
.
Takeshita, Isao; Ono, Akio; Izawa, Naoki*; Miyoshi, Yoshinori; Maeda, Atsushi; Sugikawa, Susumu; Miyauchi, Masakatsu
Proceedings of 6th International Conference on Nuclear Criticality Safety (ICNC '99), p.1512 - 1576, 1999/09
no abstracts in English
Sagawa, Norihiko*
PNC TJ9613 97-001, 90 Pages, 1997/10
An ionization sensor. which ionizes iodine vapor on a heated filament and collects ionized iodine on a collector at positive potential, was made on an experimental basis. Iodine vapor in rare gas was determined with using the sensor on the line and the characteristic of the sensor was examined. Iodine vapor was generated from iodine crystals in an iodine evaporator and transferred to the sensor with carrier-rare gas. The iodine vapor was continuously monitored by the sensor and trapped in solution of sodium hydroxide. The amount of iodine in the solution was determined by chemical analyses. The integrated value of ion current was compared with the collected amount of the iodine. A proportional relation is observed between the collected amount and the integrated value obtained from the sensor with a platinum collector, but not found between the amount and the value obtained from the sensor with a stainless steel collector.
Homma, Shunji*; Tajima, Yasunori*; Koga, Jiro*; Matsumoto, Shiro*
PNC TJ1609 97-001, 47 Pages, 1997/02
no abstracts in English
Nishio, Gunji; Koike, Tadao; Miyata, Teijiro; Takada, Junichi; *
JAERI-Tech 95-029, 59 Pages, 1995/03
no abstracts in English